TENDOCOLINN®

TENDOCOLINN® is a dietary supplement with specific bioactive collagen peptides obtained from hydrolysed bovine collagen, dry extract of acerola fruit and vitamin B6. Bioactive collagen peptides in the patented formulation Tendoforte®B stimulate the creation of collagen, proteoglycans and elastin, which provides for firmness, strength, better mobility and elasticity of tendons and ligaments.
Why TENDOCOLINN®?
- It restores the strength and elasticity of tendons and ligaments
- It provides for firmness, strength, better mobility and elasticity of tendons and ligaments
- It has anti-inflammatory effect
- It speeds up the process of recovery and return to daily activities and training
- It reduces the risk of new injuries
- Safe for long-term continuous use
- It has a pleasant taste

In which conditions is the administration of TENDOCOLINN® recommended?
- For treatment of tendon and ligament injuries and for prevention of new injuries in athletes and people doing sports for recreational purposes
- To people with chronic ankle instability
- To persons with acute and chronic inflammation of the ligaments and tendons (e.g. the Achilles tendon)
- To obese persons with increased strain on the joints and ligaments
- To the elderly
What does TENDOCOLINN®contain?
Active ingredients | 1 sachet |
|---|---|
Tendoforte®B (bioactive collagen peptides)
| 5 g |
Dry extract of acerola fruit
| 120 mg
|
Vitamin C
| 30 mg
|
Vitamin B6 | 1,3 mg
|
TENDOFORTE®B
High single dose of specific bioactive collagen
peptides restores
the structure of tendons
and ligaments, which speeds up
the process of recovery after injury,
maintains the function of ligaments
and tendons, reduces the risk of
recurrence of injuries in the form of
sprains.
TENDOFORTE®B
High single dose of specific bioactive collagen
peptides restores
the structure of tendons
and ligaments, which speeds up
the process of recovery after injury,
maintains the function of ligaments
and tendons, reduces the risk of
recurrence of injuries in the form of
sprains.
VITAMIN C
Obtained from acerola, it contributes
to the normal synthesis of collagen
required for normal function
of cartilage, tendons, ligaments
and bones. It hydroxylates
the amino acids that make up
the collagen fibres,
thus providing
the strength and elasticity
of the newly formed collagen fibres.
TENDOFORTE®B
High single dose of specific bioactive collagen
peptides restores
the structure of tendons
and ligaments, which speeds up
the process of recovery after injury,
maintains the function of ligaments
and tendons, reduces the risk of
recurrence of injuries in the form of
sprains.
VITAMIN B6
Coenzyme in over 150 enzymatic
reactions in our body playing
a significant role
in the metabolism of proteins and
carbohydrates, neutralizing the effect
of free radicals and reducing
the inflammation that accompanies sprains,
ruptures and other injuries to tendons and
ligaments.
TENDOFORTE®B
High single dose of specific bioactive collagen
peptides restores
the structure of tendons
and ligaments, which speeds up
the process of recovery after injury,
maintains the function of ligaments
and tendons, reduces the risk of
recurrence of injuries in the form of
sprains.
Tendoforte®B – clinical studies
-
Conclusion:
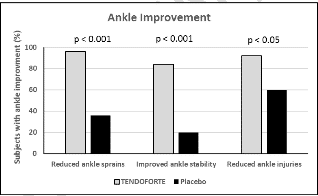
- Supplementation with the product Tendoforte®B has a positive impact on the health of ligaments and tendons.
- Clinical studies confirm that daily dose of 5 g of specific bioactive collagen peptides leads to significant improvement of condition of the patients with ligaments and tendons problems.
- There was a statistically significant improvement of ankle stability in athletes with chronic instability of an ankle and the risk of repeated injuries in the form of sprains was reduced (Dressler et al., 2018).
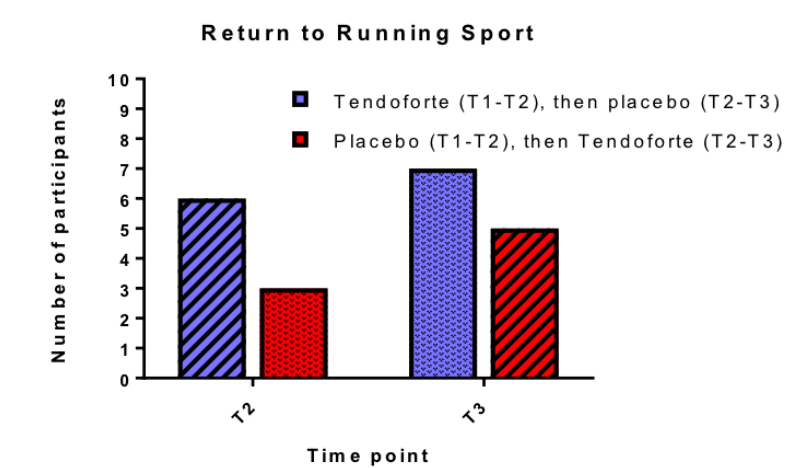
- Clinical data of the Australian Institute of Sports (AIS) demonstrated that athletes with the Achilles tendon problems, who were not able to run and did not respond to any of the traditional therapy, could resume running within a shorter time using only 5 g of product Tendoforte®B daily during 3 months.
Method of use
Dilute one sachet of powder a day in 200 ml of fluid and drink it.
Presentation: 28 sachets.
Flavour: orange.